
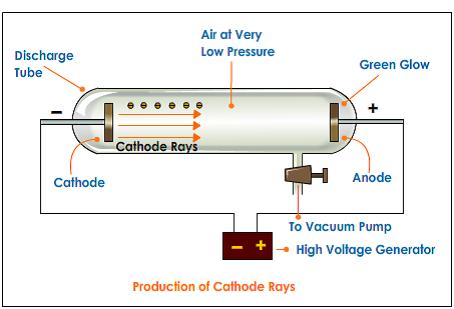
Thomson measured the deflection of the beam using a ruler etched on the end of. You can see a simulation of this glow on the far right of the applet diagram, as shown in Figure 2. The present paper aims to use paradigmatic responses to Galison’s problems to explore the differing natures, merits and limitations of these two paradigms. In Thomson’s experiment, a fluorescent material was coated on the end of the tube to produce a glowing dot where the cathode rays hit. The paper argued for recognising at least two paradigms, one based on logic, and analytic forms more generally, the other based on deliberative judgement making. Thomson had shown that cathode rays behave as one would expect negatively charged material particles to behave. In a recent paper Hooker (Perspect Sci 26(2): 266–291, 2018b) proposed that the discipline(s) of HPS should themselves also be understood to employ paradigms in HPS to understand science, analogously to those employed in science to understand scientific domains. Thomson experimenting with cathode ray tubes. Cathode ray experiment was a result of English physicists named J. Thomson, led to the discovery of the negatively charged part of the atom, the electron. Thomson Experiment The Discovery of Electron.
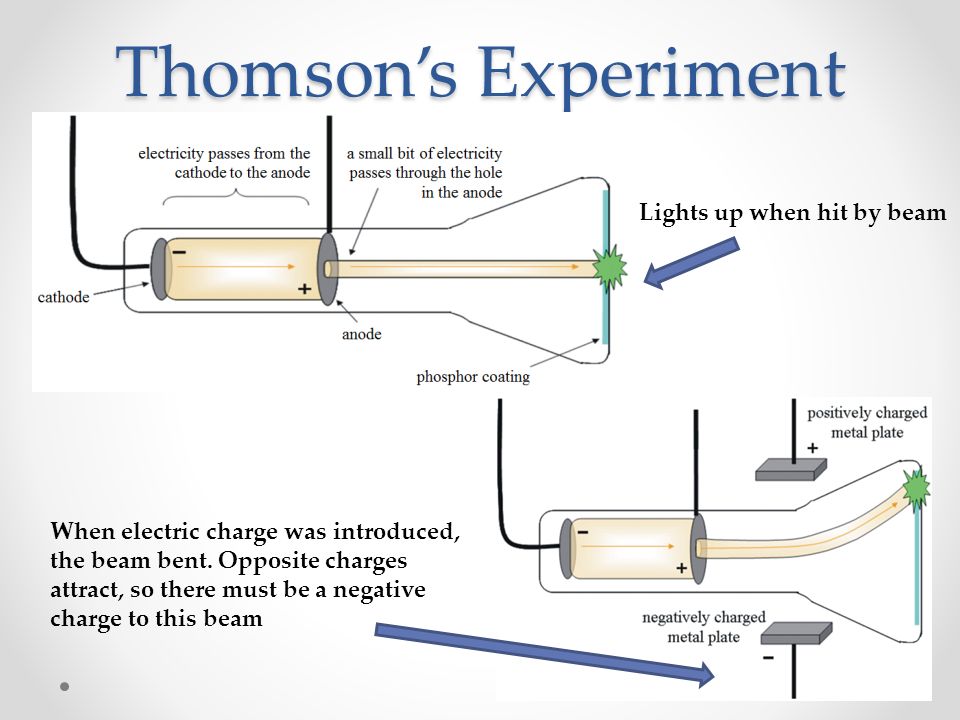
Recent research provides a basic resolution of these issues. The cathode ray tube experiment, originally carried out by J.J. It is however unclear to what extent these problems, and constraints on their solutions, are of HPS’s own making. Is exploited in making powerful tools for the exploration of the atomic world, like the mass spectrometer.In an Isis 2008 review of research in History and Philosophy of Science (HPS), Galison opened discussion on ten on-going HPS problems. The force that moves electrons through the vacuum tube is an essential force that mediates all important interactions in chemistry. The cathode rays that impact the butterfly cause it to fluoresce, just as Thomson's screen was made to fluoresce in his classic experiment. Within the tube is a butterfly that is painted with fluorescent compounds. In our cathode ray tube, we run electrons - or cathode rays - through the tube. The cathode sprayed out its "rays" and those not absorbed by the anode would illuminate the screen, leaving a shadow of the cross-shaped. Beyond the anode was a fluorescent screen covered with zinc sulfide. Thomson proposed the plum pudding model of the atom, which had negatively charged electrons embedded within a positively charged soup. Thomson (1856-1940) the electrons were introduced at one end containing the cathode and collected in the middle by a cross-shaped anode. Thomsons experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson discovered the electron by using a cathode-ray tube, He. In between, the rays encounter a fluorescent screen, causing it to glow. watch JJ Thomsons cathode ray tube experiment. The charged electrons in the electric field feel a force that causes them to flow from the cathode toward the anode where many are captured. The voltage placed across the tube creates an electric field. The electrons are introduced to the vacuum tube by the cathode. In Thomson’s experiment, a fluorescent material was coated on the end of the tube to produce a glowing dot where the cathode rays hit. The mysterious "cathode rays" flowing through the vacuum tubes are now known to be currents of electrons. Cathode ray tube (CRT) - a vacuum-sealed tube in which electrons flow from the cathode. During his experiment he discovered electrons and it is one of the most important discoveries in the history of physics. Thomson - an English physicist who used cathode rays to discover the existence of electrons in 1897.
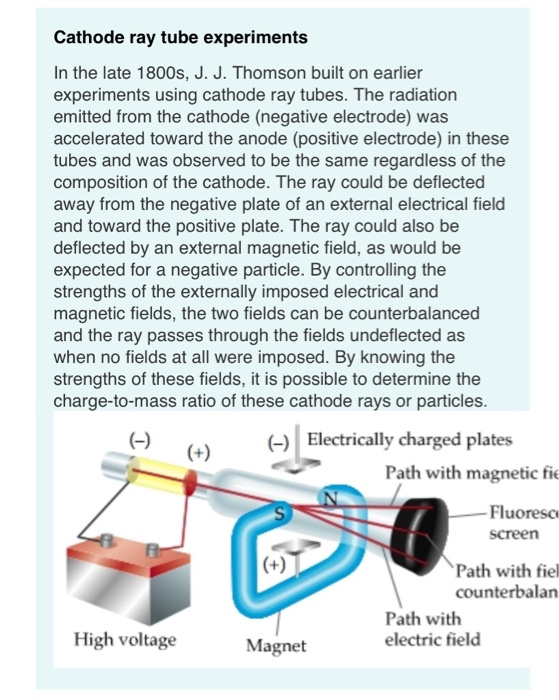
Apply voltage to end of cathode ray tube. In Thomsons experiment, which was used to determine the velocity of electrons, an electric field E is applied between two metal. The Cathode ray experiment was a result of English physicists named J. Voltage is applied across a vacuum tube creating an invisible beam of "cathode rays" that magically illuminate a fluorescent screen.ġ.


 0 kommentar(er)
0 kommentar(er)
